Abstract
As clinical applications for Chimeric Antigen Receptor (CAR) T-cell therapy extend beyond early phase trials, larger scale cell manufacturing processes incorporating cryopreservation steps become a logistical necessity. Mononuclear cells (MNC) collected by leukapheresis, which serve as the pre-culture "starting" fraction (PeC) for CAR T-cell production, may need to be cryopreserved prior to cell transportation to a centralized manufacturing facility. PeC aliquots may be cryopreserved for repeat cultures in cases of primary culture failure or for patient re-dosing. Further, cryopreservation of the post-culture (PoC) CAR T-cell product is essential in order to ease post-manufacture transportation, ensure completion of regulatory testing prior to product release and avoid product wastage due to unanticipated patient-related delays. The effect of cryopreservation on CAR T-cell viability, expansion, phenotype and transduction efficiency merit evaluation prior to routine use.
From 2012 to 2017, data was obtained on 160 consecutive autologous CAR T-cell cultures initiated for patient infusion on 6 clinical trials of different CAR T-cell types (1. Anti-CD19-pediatric trial (n=58), 2.Anti-GD2 (n=14), 3.Anti-BCMA (n=21), 4.Anti-CD22 (n=43), 5. Anti-CD19-adult trial (n=22), 6. Anti-CD30 (n=2)).Following autologous leukapheresis collection, PeC was either cryopreserved-thawed (PeC-C) or used fresh (PeC-F) for CAR T culture initiation, depending on patient needs. Thereafter, cell selection, transduction-expansion and harvest were performed according to trial protocol specific instructions. The harvested PoC was again cryopreserved (PoC-C) or infused fresh (PoC-F). Data on patient demographics, number, timing and duration of cryopreservation, Total Nucleated Cell (TNC) count, viability, fold expansion (FE), transduction efficiency (TE), CD3% and CD4/CD8 ratios was collected. Post thaw TNC recovery (PTR) % was calculated as TNC*viability at thaw/TNC* viability at cryopreservation.
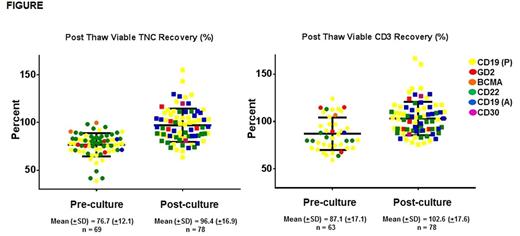
Afterdiscarding 13 products due to manufacturing failure (MF) during culture, 147CAR T infusates were prepared for pediatric or adult patients with hematologic malignancies (135, 91.8%) or solid tumors (12, 8.2%). Mean age was 27.7 years (±19.9), weight 60.7 kg (±26.3), median CAR T-cell dose of 1x106 CAR-transduced viable CD3+ cells/kg (Range: 1x105 - 1x107). Gamma-retroviral or lentiviral vectors were used for CAR delivery in 88 (59.9%) and 59(40.1%) infusates, respectively. Pre-culture lymphocyte concentration included ficoll alone (42, 28.6%), flask adherence and/or elutriation (14, 9.5%), CD3.CD28 and/or CD4/CD8 selection (68, 46.3%). Of the 147 infusates, PeC-C and PoC-C were 69 and 78, respectively. Median duration of PeC-C and PoC-C was 6 days (Range: 3-868) and 9 days (Range: 1-408), respectively. TNC and CD3 PTR were lower in PeC-C than in PoC-C (Figure). At harvest (PoC), Mean FE, TE, CD3% and CD4/CD8 ratio were 12.8 (±12.5), 54.3 (±21.9), 97.6 (±4.4) and 1.9 (±2.0) respectively. In multivariate analyses, FE, TE, CD3% and CD4/CD8 ratios at harvest were unaffected by PeC-C status or duration. TE differed by trial. Twelve out of 13 (92%) MFs were due to failure of PeC transduction and/or expansion. Of the 12 MFs, 6 products were PeC-C (50%); 6 were PeC-F (50%). Among the 6 PeC-C MFs, none of the failures could be attributed to cryopreservation. In fact, 4 of the 6 PeC-C cultures were re-initiated with an additional PeC-C and all 4 yielded a successful product.
Pre-culturecryopreservation impacts viable TNC and CD3% recovery during CAR T-cell manufacture compared to post-culture cryopreservation. Within this PeC fraction, CD3+ cells appear to have better viable recovery than the whole TNC fraction. This is likely due to a higher (differential) loss of monocytes and NK cells within TNCs. Despite these findings, most products manufactured after initial cryopreservation met clinical dose requirements. FE, TE, CD3% and CD4/CD8 ratios were unaffected by initial cryopreservation. The post thaw viability of CAR T-cells cryopreserved post culture was greater than cryopreserved peripheral blood CD3+ cells. Cryopreservation at either end of CAR T-cell manufacture is a viable strategy and may ease logistical constraints associated with large scale cell production. If optional, CAR T-cells cryopreserved post manufacture may be preferable to cryopreserving the starting fraction.
Kochenderfer: Bluebird bio: Research Funding; Kite Pharma: Research Funding; N/A: Patents & Royalties: I have multiple patents in the CAR field.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal